ALA’s Positive Pre-IND FDA Meeting
Big milestone ticked off…
Human trials are approaching…
Our cancer fighting biotech, Arovella Therapeutics (ASX: ALA) has been one of our best performing Investments.
And today we learned that ALA received positive feedback from the US FDA regarding its plans to commence a Phase 1 clinical trial for its lead cancer cell therapy candidate ALA-101.
At this Pre-IND meeting the FDA provided guidance on the company's chemistry, manufacturing controls, non-clinical studies, and proposed trial design.
Investigational New Drug submissions are required to start a Phase 1 trial in humans - and ALA plans to submit in "early Q1 2025". (Source)
ALA has previously shared promising data from trials in mice.
As you can see below, ALA’s therapy on the far right hand side (CAR19-iNKT), killed the cancer faster (removed the coloured blobs) than other similar treatments:
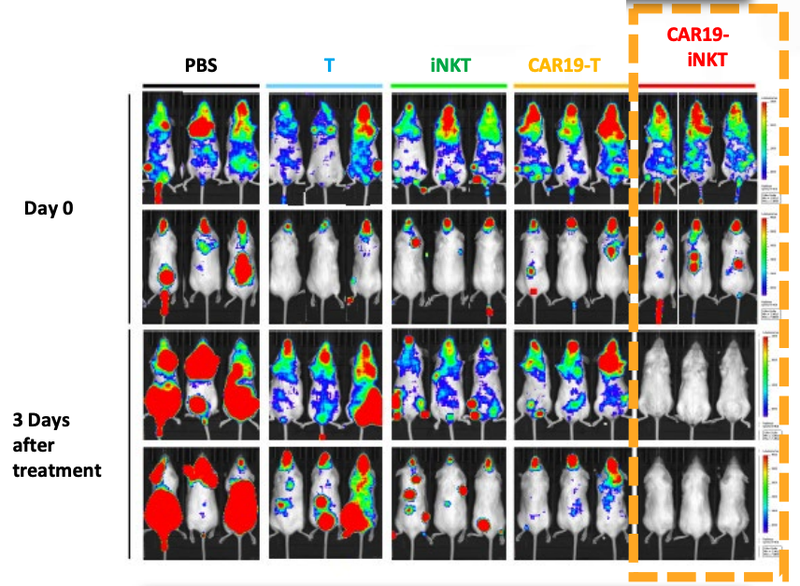
Also, ALA’s treatment had a higher overall survival rate compared to T cell therapies.
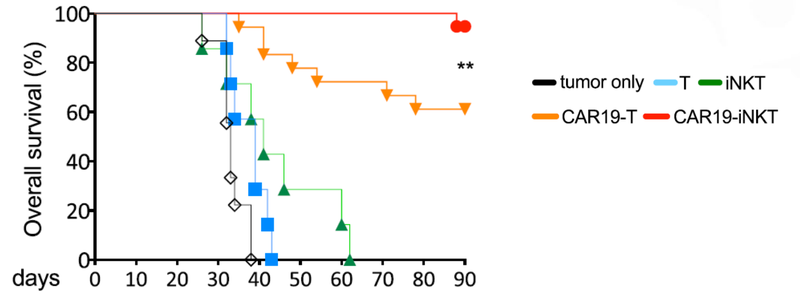
This graph shows that at 90 days ALA’s treatment had >90% survival rate, compared to 60% survival rate for T cell therapy:
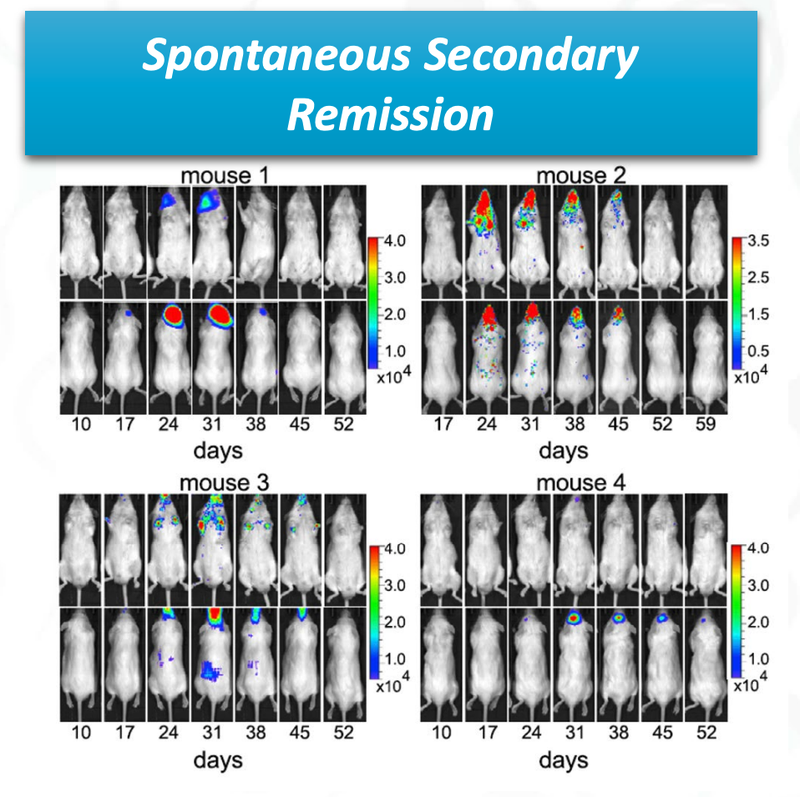
The final promising data from ALA’s preclinical trial is that there was “spontaneous secondary remission” from a number of mice.
You can see from the image that over time the cancer cells (coloured blobs) come back, but then are soon after destroyed.
So this Pre-IND meeting marks an important milestone for ALA, as it looks to advance its lead therapy, ALA-101 into human trials.
We covered the data above, and the growing potential of ALA’s cell therapy platform in our latest ALA note:
While we think today’s news means ALA’s Phase 1 trial might not happen this year as previously expected, we’re not concerned that the timeline has shifted back slightly to Q1 2025.
This is the type of important work that is best done once and properly.
How does today’s news impact our ALA Investment Memo?
Objective #1: Prepare for Phase-1 clinical trial for treatment #1
Preclinical studies have been completed for ALA’s first treatment (CAR19-iNKT) and we expect ALA to spend the next 12-18 months preparing for a Phase I clinical trial.
This involves completing the manufacturing milestones for the treatment, completing the trial design and securing ethics approval.
Source: 18 February 2022 ALA Investment Memo
Today’s news marks further progress towards ALA starting it’s first Phase 1 trial.
Objective #3: Explore complementary licensing opportunities
ALA’s board has deep knowledge of the cancer immunotherapy market. If a compelling opportunity to licence further tech for their portfolio emerges, we want to see ALA expand on their existing base of technology.
Source: 18 February 2022 ALA Investment Memo
We also note that ALA has made good progress on complementary licensing opportunities giving it a clinical pipeline brimming with additional opportunities.
In other words, ALA has plenty of shots on goal in addition to its upcoming Phase 1 trial for blood cancer.
What’s next for ALA?
In the company’s latest quarterly, ALA provided a good summary of what is needed to achieve the milestone of first patient dosed:
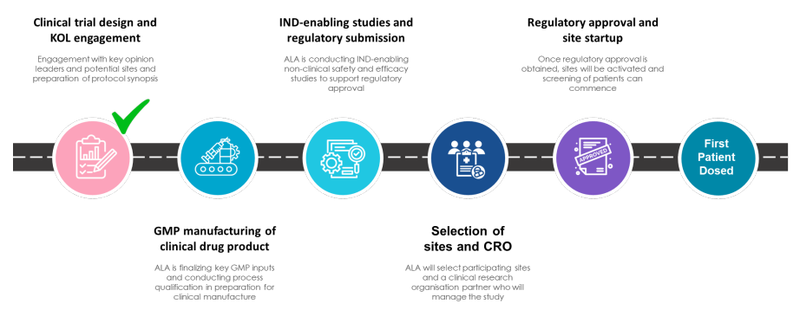
As a result we’re looking for:
🔄GMP Manufacturing
🔲IND-enabling studies and regulatory submission
🔲Selection of sites and Contract Research Organisation (CRO)
🔲Regulatory approval and site start up
🔲First patient dosed






